Real-world data (RWD) and real-world evidence (RWE) are playing an increasing role in health care decisions.
DATA-CAN has developed a RWD Service which works with public sector, academic or commercial partners to conduct analysis with data that is part of routine care systems, within the NHS of each of our data partners. Each project is approved by appropriate NHS entities, as our main objective is to improve patient care using data. By producing high impact research and support efficient health care decision making.
This work is the result of a close collaboration between several NHS hospitals. The following data is available:
- Demograhics
- Diagnosis and pathology details
- Localised treatment details
- Biomarkers
- Outcomes and follow up
- Healthcare utilisation
Data around responses and adverse events will likely require manual curation against medical notes. We can support you through the development of the project’s protocol, as data and clinical experts.
Patient-level data remains within the NHS secure environment throughout the study, and all analysis takes place by NHS staff. The data is de-identified prior to analysis to further protect patient privacy. The requestor does not access patient data at any point (if this is something you are interested in, please contact our SDE team). Aggregate reports where small numbers are hidden will be released from NHS, subject to censoring for NHS disclosure control of the participating data partners.
We support studies investigating: time to treatment analysis, treatment patterns, survival, incidence, prevalence… See previous projects here.
How does it work?
Our experienced team of medical oncologists, analysts, data managers and coders provide high quality work based on clinical and research expertise. We will support you through each step of the analysis, as described below:
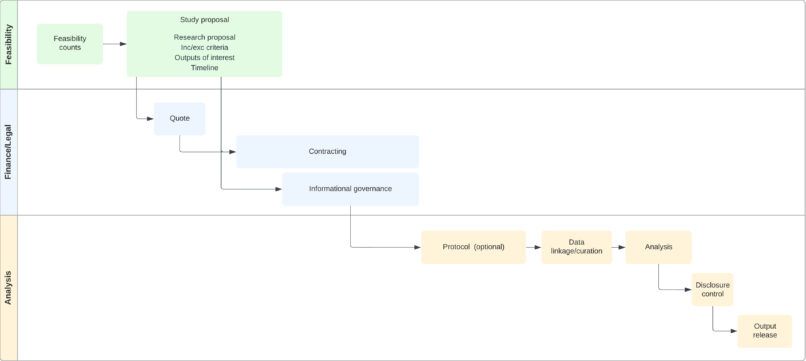
1- Feasibility – data exploration
This is an interactive process where we would share our expertise (both clinical and analytical) and support you in the development of your study concept and feasibility. Get in touch with information about the cohort you are interested in, and we can investigate if we have enough numbers and variables to power the analysis. Please complete this form as you get in touch.
2- Finance/Legal work
If you approve of the above, a quote will be provided based on a cost-recovery model, with an anticipated delivery time-frame. Once approved, a contract will be put in place.
Information governance approval will be requested by our team if this quote is approved. This process is necessary to maintain patient data privacy, and is expected to last about 12 weeks.
3- Analysis
A protocol, created with the requestor, can be developed. We would organise 2-3 rounds of review, and can use the requestor’s template if requested. The analysis is then performed by an NHS analyst, on an NHS computer, on the NHS IT environment. The data does not leave this set up. All our staff are trained for data protection as well as for the NHS disclosure policy. Shell tables will also be created. The requestor will not have access to the patient data (if this is something you are interested, please contact our SDE team). A draft of the output (aggregated data only), will be submitted to the NHS Lothian Caldicott Guardian, via the Dataloch team, for final disclosure control as per NHS policy. This is to ensure that the data shared does not allow for patient identification, and that small numbers are hidden. The output is then shared with the requestor.
The output is a product of collaborative work and is published in the public domain for use as part of an evidence based technology development and adoption process.
Data quality assurance
With real-world studies, missing or inaccurate data are expected in line with the nature of routine electronic health records. Critical variables are identified during protocol development and data completeness will be reported for these variables.
Manual data curation can be undertaken to complete critical missing values and to quality check accuracy using a sample-based approach. Where critical missing data cannot be completed patients will be excluded from the analysis.
Our studies are conducted in accordance with the ethical principles that have their origin in the Declaration of Helsinki and that are consistent with Good Pharmacoepidemiology Practices (GPPs) and applicable laws and regulations of the country or countries where the study is being conducted, as appropriate.
If you are interested in commissioning an analysis please contact maheva.vallet@ed.ac.uk.
